This page includes information pertaining specifically to the reproductive medicine industry. Learn about all the issues related to donor conception, including what's new in the industry and the related legal implications.
A note regarding the right to test your own or your child's DNA: No sperm bank has ever sued anyone for testing their own or their child's DNA. No sperm bank has ever sued anyone for reaching out to their own or their child's genetic relatives, including formerly "anonymous" donors. Thousands of donor families have connected via DNA websites since 2005 and only one was threatened with a fine, mother Danielle Teuscher. She then sued the sperm bank (California Cryobank/NW Cryo) and ended up walking away with a $75,000 settlement. See the related 2019-2020 Teuscher lawsuit documents below, along with the CBS News story, and the attorney's Q&A/case summary.
A note on the doctors who have been caught using their own sperm to impregnate their patients: While there are many more cases that we know about, beginning in the 1940s and ending in the late 1980s to early 1990s, here is a short list of the public cases involving doctors who have deceptively used their own sperm to impregnate their patients:
Hal C. Danzer, Los Angeles Fertility Institute
Patrick Steptoe, UK
Dr. Charles Peete, Duke University Hospital
Dr. Quincy Aselli, Indianapolis, IN
C.F. Douglas Ackman, McGill Clinic, Montreal
Martin Greenburg, NY
Marvin Yussman, KY
Gerald Mortimer, Idaho
Donald Cline, Indianapolis, IN
Cecil Jacobson, Fairfax, VA
Roger Abdelmassih, Brazil
Ben Ramaley, CT
Narendra Tohan, CT
Norman Barwin, Ottawa
Bertold Wiesner, UK
Thomas Lippert, Utah (convicted kidnapper)
Norman Tony Walker, South Africa
Jan Karbaat, Barendrecht, the Netherlands
Jan Beek, The Netherlands
Henk Nagel, The Netherlands
John Boyd Coates III, VT
Dr. Katzorke, Germany
Paul Jones, CO
Kim McMorries, TX
Michael Kiken, VA
Phillip M. Milgram, CA
Jan Wildschut, Zwolle, The Netherlands
Gary Vandenberg, CA
Quincy Fortier, NV
Philip Peven, MI
David Claypool, Spokane, WA
Morris Wortman, Rochester, NY
Gary Phillip Wood, AR
James Blute III, AZ
Edwin Delfs, CA
Gary Don Davis, ID
Sidney Yugend, IA
Robert Tichell, NY
Dr. Burton Caldwell, Yale-New Haven Hospital, CT
Dr. Joseph B. Forman, Yale-New Haven Hospital, CT
Dr. John G. Marsh, Orlando, FL
Dr. John Doherty, Sydney, Australia
Dr. Christopher Herndon, WA
Dr. Merle Berger, MA
Dr. Gottlieb, Solon, Lenox Hill Hospital & the Margaret Sanger Clinic in NYC.
A paper on "open" or "willing to be known" donors. Our white paper. The Ambiguity of Open Gamete Donation.
Bills Introduced and/or Passed
March 2025: Oregon Senate Bill 163
Requires the Judicial Department to study the judicial and administrative procedures to establish parentage and changes that would be necessary to implement the Uniform Parentage Act in this state.
Senate Bill 163 would require clinics that collect sperm, egg or embryo donations to ask for the names, birth dates, addresses, phone numbers, emails and medical histories of donors, which would then be entered into a state donor registry where the information would remain even if the clinics went out of business. When the children born from those donations turn 18, they would be entitled to access their donors’ information.
Robin Pope, a Portland lawyer who specializes in assisted reproductive technology law, told the Senate Judiciary Committee Monday the idea is not that much different from the rights granted to adoptees. In 1998, Oregon voters approved Ballot Measure 58, which enables children who were adopted, upon turning 21, to receive an unsealed birth certificate that includes the name or names of their parents. Senate Bill 163 goes a few steps farther by calling for the release of more information and at an earlier age, 18 instead of 21.
February 2025: Arkansas State Legislature HB1554
TO CREATE THE ASSISTED REPRODUCTIVE TECHNOLOGY REPORTING ACT. The federal Fertility Clinic Success Rate and Certification 29 Act of 1992 requires the Centers for Disease Control and Prevention to track 30 certain health outcomes and success rates of assisted reproductive 31 technology; 32 (2) However, this law lacks a strong enforcement mechanism, and 33 is too limited in scope; 34 (3) Additionally, the law governing assisted reproductive 35 technology in Arkansas does not require fertility clinics to report key data 36 HB1554 2 02/21/2025 1:40:08 PM JMB056 points related to assisted reproductive technology, maternal and neonatal 1 health, and the total number of embryos created through this procedure; and 2 (4) Therefore, many prospective parents, lawmakers, researchers, 3 and fertility clinics lack an adequate understanding of how assisted 4 reproductive technology functions in the State of Arkansas and information 5 that is essential for prospective parents as they make important decisions 6 about their childbearing options.
February 2025: Colorado House Bill HB25-1259
An industry-backed bill (California Cryobank) that would gut Colorado’s Donor-Conceived Persons and Families Protection Act (DCPPA). This bill weakens protections implemented in Senate Bill 224. 3/13/25 Aspen Public Radio: Colorado lawmakers consider rollback of sperm donor disclosure requirements adopted in wake of scandals.
May 2025 this bill passed. Fertility Donor Bill Heads to Governor’s Desk
January 2023: Maryland House Bill 482 :
The bill proposed bringing the minimum donor age to 21 years old, limiting the number of families per donor to 25, and would give a donor-conceived person access to their donor's identity and medical history when they turn 18.
June 2022: Fraud in Assisted Reproduction Act passed in Iowa.
June 2022: Iowa Bill 529: Fraud in Assisted Reproduction Act.
May 2022: Senate Bill 224 passed in the Colorado legislature.
DSR COMMENTARY:
The bill prohibits gamete vendors in the state of Colorado, and out-of-state gamete vendors selling gametes to Colorado recipients from being permitted to facilitate an anonymous donation.
But....all sperm and eggs are and will still be sold as anonymous, be it for 18 years or forever. When the first donor child found their biological father via a DNA test in 2005, anonymity should have no longer been promised or mandated. Why continue to perpetuate the deception and false narrative of 18 years of anonymity? And...nothing changes for the millions of donor-conceived people already born or for those who will be born in the next 3 years. We won’t know if anything has really changed until the year 2043 when the first affected donor children turn 18.
The bill states that any donor will be required to consent to the release of their medical history when any donor-conceived person, conceived using that donor’s gametes, reaches the age of 18.
But... since the advent of sperm banks, for many decades, families have been receiving self-reported non-identifying medical information from donors on their donor profiles. This bill doesn’t mandate collecting, updating, or sharing new medical information with the families. So what changes?
The bill stops egg and sperm banks from permitting more than 25 family recipients for any given donor’s gametes. For counting purposes, the 25 families are based on “live births,” do not limit a family from additional siblings, and do not count family-to-family embryo donation.
But...how? You can’t set “limits” until there is accurate record keeping, which doesn’t exist and can not without mandatory birth reporting. Facilities do not know how many children are born from any one donor. Many egg and sperm donors donate to more than one clinic.
So who will mandate, oversee, or pay for the new accurate record-keeping between now and 2043?
The answer: no one.
March 2022:Two bills recently introduced in Michigan make it a felony to provide false or misleading information related to an assisted reproduction procedure and permit victims (including offspring) to file suit for damages resulting from such information.
http://legislature.mi.gov/doc.aspx?2022-HB-5714
http://legislature.mi.gov/doc.aspx?2022-HB-5716
Recent Media: What's New in the Donor Conception Industry?
November: Belga News
Health minister proposes end to compulsory anonymity in sperm and egg donation
Federal Health minister Frank Vandenbroucke wants to abolish compulsory anonymity in sperm and egg donation in Belgium. Under a proposal he has submitted to the government, donor-conceived children would gain the right to information about their donor from the age of 12.
The age of 12 is a lower threshold than in many other countries, where the limit is 16 or 18. Vandenbroucke said he chose this age because Belgian children then gain more legal autonomy: they can be questioned within youth welfare or family court, and they can authenticate themselves using their identity card.
October: Local 3 News/CNN
September: 7 News
Queensland IVF company admits to sperm donation error after bi-racial baby born to Caucasian couple
The company acknowledged how the mistake was made.
QFG, which has been under new ownership since 2022, said it cut ties with Seattle Sperm Bank, which took full responsibility for the incident.
June: Star Observer
Mothers Are Calling for Urgent Reform After IVF Error Leads To Youth Suicide Attempt
Ms Gunn, whose family has been at the centre of an IVF baby swap scandal, claimed her son’s suicide attempt was directly tied to the discovery that he and his siblings were not fully related by blood, a revelation that emerged through DNA testing after the children developed serious health conditions.
“We want the minister to realise that laboratory mistakes have real human impact throughout generations,” Ms Gunn told The Saturday Courier Mail. “We want everyone to know that.”
Her son, who suffers from a host of chronic medical issues including hypermobile Ehlers-Danlos syndrome, severe chronic fatigue, and autism spectrum disorder, now battles long-term chronic pain.
Ms Gunn and her partner, Lexie, are currently suing Queensland Fertility Group over claims that the wrong sperm donor was used in the conception of two of their three sons.
The emotional and physical toll has been immense for their child, who has struggled not only with chronic pain but with the emotional impact of his disrupted genetic identity, so much so that they claim he attempted to take his own life.
“Imagine a boy that young being so sad, not only about his physical pain but the fact that through DNA testing he found out that all three brothers were not fully connected with the same blood,” Ms Gunn said.
“That has been so hard on him, he feels a deep sense of lost familial connection.”
From now on, every person conceived via sperm or egg donation will have the legal right to access information about their biological donor once they turn 18.
It’s a step forward in terms of transparency and personal identity. Starting with children conceived from 2022 onwards, the new rule means that once they reach adulthood, they’ll be allowed to request both identifying and non-identifying details — such as the donor’s name, age, appearance, health status, profession, family situation, and even a personal letter if the donor wrote one.
US sperm donor giant California Cryobank is warning customers it suffered a data breach that exposed customers' personal information.
"Through our investigation, CCB determined that an unauthorized party gained access to our IT environment and may have accessed and/or acquired files maintained on certain computer systems between April 20, 2024 and April 22, 2024," reads a data breach notification from California Cryobank.
The investigation has determined that the attack exposed varying personal data for customers, including names, bank accounts and routing numbers, Social Security numbers, driver's license numbers, payment card numbers, and/or health insurance information.
Sperm bank breach deposits data into hands of cybercriminals
Donor-conceived South Australians will now have greater access to knowledge about their biological parents – even in cases where donors were previously guaranteed anonymity.
SA Health on Wednesday launched a new South Australian Donor Conception Register, an online site where donor-conceived people will be able to access some information about their biological parents.
“In a country of 340 million people, we only have 1,200 donors”
— Dr Brian Levine
SAVANNAH, Ga. (AP) — Krystena Murray became pregnant after undergoing in vitro fertilization two years ago, and said she was unaware until she gave birth that the fertility clinic had made a fateful mistake.
Coastal Fertility Specialists operates a clinic in Savannah and four others in neighboring South Carolina. The medical practice apologized in an emailed statement for what it called “an unprecedented error that resulted in an embryo transfer mix-up.” It said the staff has adopted new safeguards to prevent similar mistakes from happening in the future.
An alternate path to parenthood that has endowed families around the world, donor conception raises a multitude of questions about identity, genetics, and ethical considerations. This week on All Together: The Family Science Insights Podcast, host Marie Stella is joined by Wendy Kramer, the co-founder and director of the Donor Sibling Registry, to explore these questions and more. Founded in 2000, Donor Sibling Registry was founded with Wendy Kramer’s donor-conceived son Ryan, to assist individuals conceived as a result of sperm, egg, or embryo donation, who are seeking to make mutually desired contact with others with whom they share genetic ties.
With over 95,000 members in 105 countries, the DSR has helped connect more than 26,250 people with their half-siblings and/or biological parents. Wendy Kramer has listened to, advised, consulted, and researched thousands of these parents, donors, donor-conceived people, and other donor family members. In this episode, Wendy Kramer asserts that the reproduction medicine industry is highly unregulated, with much information supplied by sperm banks or donor clinics being misinformed as part of marketing efforts to draw in more clients. She reveals that in the digital age, the ‘anonymity’ typically promised by such clinics is near-impossible to achieve, with social media platforms and DNA tests readily available for public consumption. Wendy Kramer cites her son Ryan as an example of a donor-conceived child being able to locate their biological parent all on their own.
Together, Wendy Kramer and Marie discuss the importance of open conversations in donor-conceived families, from when to disclose such information to a child, to various ways to normalize these conversations. So, whether or not you plan to embark on the route to donor conception, tune in for an incredibly eye-opening episode!
In a precedential ruling, a Family Court in Israel held that the genetic progenitors of a two-year-old child born to a genetically non-related couple following a high-profile embryo mix-up at a fertility clinic, shall be determined her parents.
Wolf, who specializes in suing fertility clinics that have been responsible for life-altering errors, says he had encountered fewer than 10 cases in which an embryo was transferred to the wrong woman. But he estimates that over the past decade, he has represented more than 1,000 plaintiffs accusing clinics and their suppliers of misconduct or negligence, most commonly because embryos in their care have been accidentally lost, damaged or destroyed.
The Connecticut Supreme Court faces philosophical questions about the value of life this week when it hears an appeal by two people who claim they learned as adults that they were conceived after a reproductive health specialist treating their mothers substituted his sperm for that of their fathers.
The bond between siblings is too important to not be explicitly recognised as a human right. The law needs to recognise the right of siblings to stay together, reunite if they’re separated, and identify and connect with each other in the case of genetically-related siblings raised in different families.
Kramer, who still runs the Donor Sibling Registry — it now has more than 92,000 members — isn’t impressed with the promise of revealing information when a child turns 18. She believes that making kids wait is harmful. “Nowhere ever in history, anywhere on the planet,” says Kramer, “has anyone deemed it in a human being’s best interest to be kept from their close genetic relatives for the first 18 years of their life.”
"It is the first law of its kind in the U.S. Australia and a number of European countries already prohibit anonymous sperm and egg donations, giving donor-conceived people access to more information about their identities and family histories."
This is inaccurate as those donor-conceived people are conceived with US and Danish donors. (As their donor pools dried up, eg. in Australia, Canada, and the UK.) So no, DCP in those countries do not "have access to more information about their identities and family histories." They're in the same position as everyone else conceived with US and Danish sperm.
Nearly half of all audited samples-about 42%-were found to have errors.
These included lost or incorrectly labeled samples and problems with storage and handling that affected sample quality. As a result, thousands of sperm donations are now set to be discarded due to these identification errors.
Wendy Kramer, director and co-founder of the Donor Sibling Registry, disagrees. “[O]ur research paints a different picture. In our first published study of 155 egg donors, we found that 30.3% reported Ovarian Hyper Stimulation Syndrome (OHSS),” she previously wrote. “In our second survey of 176 egg donors in 2014, we found that 32.4% of egg donors reported complications such as OHSS and infection. In our third Study of 363 egg donors in 2021, 22.4% reported experiencing OHSS.”
Wendy Kramer is working to change that. Her son, Ryan, was conceived with donor sperm and born in 1990. She was honest with him about his conception from an early age. When Ryan was about 6 years old, he asked to meet his biological father. The sperm bank wouldn’t share any information. So around 2000, Kramer started to connect with other people like her and Ryan to create The Donor Sibling Registry, a nonprofit organization that has enabled more than 25,000 half-siblings and/or their donors to meet to date (including many of Greene’s donor-conceived children).
In 2005, Kramer’s son took a DNA test, which led mother and son to seek out and build a relationship with his biological father. To date, Wendy and Ryan Kramer have also identified 28 half-brothers and sisters. Kramer says that many of those siblings weren’t told by their parents that they were donor-conceived and were caught off-guard when they learned the truth through genetic testing. “You get families that are imploding. Kids who are struggling. Trauma,” she says. “Some of [Ryan’s] half-siblings thought it was a prank. They deleted the emails from the half-siblings saying, ‘Someone’s pranking people in 23andMe. My parents didn’t use a donor.’”
Kramer recommends that donor parents tell their kids the truth before the child can even speak, and that sperm banks should enable connections at any age. Just as openness has risen around adoption, she’d like to see that happen with donor conception. “Then it’s just a part of their story to be proud of,” she says.
After a Connecticut woman took a DNA test, her life was turned upside down. She discovered she was likely a product of fertility fraud and that she had at least 22 half siblings, one of whom was her high school boyfriend.
A lawsuit accused the doctor of 'surreptitiously' inserting his sperm into the patient instead of a donor's.
Their donor died unexpectedly. Every time a new sibling finds them, they, the recipients — not the sperm bank, not the donor's family — have to break the news.
In June 2023, Anke Wesenbeek, a 30-year-old Belgian donor-conceived woman won a landmark court case after a year-long battle to gain information about her paternal heritage.
Both were from Utah and had sons born via IVF. But the Johnsons and the McNeils, it turned out, had much more in common than that …
Finding lots of siblings is common and a result of a lack of donor limitations, co-founder says
Wendy Kramer is a co-founder and director of the Donor Sibling Registry, a Colorado organization that helps people conceived via sperm, egg, or embryo donation find relatives. It happens every day through her organization, she said.
She has been connecting people from Siperko’s group for decades, including 38 of Siperko’s siblings. Altogether, the group has connected nearly 25,000 people with their half-siblings and biological parents. And while some people are happy to find so many siblings, there is still an underlying issue she wants to see addressed: a lack of limitations on donating sperm.
Many sperm banks initially promised that each donor would result in no more than 10 kids, Kramer said, but − evidenced by Siperko's case − that obviously didn't happen.
What sperm banks are missing, Kramer said, is "accurate record-keeping on the children born."
“That's why we have so many half-sibling groups over 100 and even over 200," she told USA TODAY. "Those groups just keep growing.”
“We all came to understand that that was a complete lie and they still lie about it,” she said, noting that in some cases, people find out they have more than 200 siblings.
Large sperm banks also ship sperm to clinics across the country and sometimes worldwide, she said. Her organization has asked donors and parents of donor-conceived people what they think the limit should be and the most common number they hear is 10.
She said it’s unethical and irresponsible for the reproductive medicine industry to create so many half-siblings groups without updating families on medical information and limiting the number of births there are from each donor.
Part of the problem, she said, is that companies need to do a better job at keeping track of births. They aren’t always reported and record-keeping isn’t as precise as it should be.
“It's just about a profit for selling sperm with no thought whatsoever given to the human beings they're helping to create,” Kramer said.
“I used California Cryobank and my son has … half-siblings coast to coast, up and down, even in Puerto Rico,” she said. “You never know where your half-siblings can be.”
What's it like to discover siblings?
How people respond when they find out they have so many siblings depends largely on the person, said Kramer. For introverts, finding out about lots of siblings could be “scarier” than it is for extroverts, she said. It’s deeply personal and there’s no telling how incorporating new people into your life will go.
But according to Kramer, there are many benefits to finding one’s siblings as well, including both medical and psychological.
“It's an innate human desire to want to know where we come from and who we come from,” she told USA TODAY. “Allowing donor-conceived people to know about their ancestry, their close genetic relatives and their family medical history is crucial.”
There are some key differences between the experiences of adopted and donor-conceived kids, but one thing remains the same: They should know about their origins.
A Southern California couple is suing a fertility clinic for fraudulent concealment claiming they mistakenly transferred an embryo carrying a rare stomach cancer gene and then falsified patient records to cover up the error.
Jason and Melissa Diaz claim their young son now faces the potential of stomach cancer or possible stomach surgery to avoid the rare cancer. They filed suit in Los Angeles Superior Court on Wednesday against Huntington Reproductive Center Medical Group, also called HRC Fertility. The couple’s doctor and IVF coordinator are also named as defendants in the lawsuit.
The report shows more than half of donated sperm used in UK fertility clinics comes from abroad.
52% of new sperm donors registered in the UK were from "donor imports" in 2020 (including 27% from the USA and 21% from Denmark)
The number of new UK sperm donors has remained consistent at around 400 a year since 2012, while new imported donors have more than doubled from almost 200 in 2013 to 400 in 2020.
Meanwhile, from late 2023 onwards, most donor-conceived people in the UK turning 18 will be able to apply to access identifying information about their donors.
However, the HFEA admits there could be problems contacting a biological parent if they live abroad.
[Yes, the DSR has been saying this for years: local laws are virtually meaningless when the donors come from the US and Denmark.]
But the US sperm-donation industry “is largely unethical and irresponsible,” according to Wendy Kramer, an American author and advocate who has been fighting for the rights of donor-conceived children for more than twenty years. Most US sperm banks promise donor anonymity, making it difficult for families who use the service to learn about health developments in their donor that may be genetic or to know about other children conceived from that donor’s sperm. Kramer, who has written extensively about the problems of donor anonymity and advocates for more medical screening, says that, with insufficient oversight and regulation of the sperm-donor industry, “money is put before the well-being of the children being born.”
In 2000, along with her son, Ryan, Kramer co-founded the Donor Sibling Registry, an organization that helps donor-conceived people track down their donors and half siblings. (The process ensures the donor and siblings agree to be contacted.) The organization has since helped more than 25,000 people in more than 100 countries contact their biological families and has allowed Ryan to contact some of his half siblings.
He was also able to find his donor through DNA testing and publicly available information. “If we had not met my son’s biological father, we would not have known about some pretty serious medical issues,” Kramer says. Now Ryan and his half siblings can watch out for signs and get annual screenings for inherited health conditions.
Three-quarters (75 percent) of sperm used in the UK is imported from overseas.
Lifting anonymity from birth would pave the way for the possibility of more contact between donors, donor-conceived people and their parents, and such a proposal would be likely to spark intensive debate.
Today, at 18-years old, Daily-Anderson has 237 half brothers and sisters that he knows of. Some live near him in Virginia, but others are spread out across the United States and the world. According to the family, all the siblings are linked to the same man who donated his sperm to Fairfax Cryobank over many years. [NOTE: the DSR connected almost 200 of these half-siblings, not a "small handful" as the article says.]
Allegations against Dr. Paul Brennan Jones surfaced in 2019, when the now-grown children of his former patients found each other through DNA tests.
On July 7, 2021, Sultan implanted what was supposed to be the couple’s embryo into the woman’s uterus during a visit to the clinic, court records state. The following month, the couple confirmed they were expecting a baby. The pregnancy progressed normally — then the couple’s obstetrician asked for the genetic tests that later revealed their DNA did not match that of the fetus, court records state.
A Florida woman who accused a Vermont doctor of impregnating her with his own sperm instead of a donor’s in 1977 was awarded $5.25 million by a federal judge on Wednesday.
In 1989, Norman Barwin, a respected fertility doctor, helped my mother get pregnant with donated sperm. Decades later, we discovered that Barwin was secretly inseminating patients with sperm that he had no right to use, including his own. How I finally learned the truth about my biological father.
The family has sued Nicholas Spirtos, the fertility specialist who performed the procedure in 1991, and the Ohio-based Summa Health System.
In May of 2020, Laura and Dave Gunner received the worst news parents can get: their son, Steven, had died of an accidental opioid overdose. He was 27. While the Gunners were devastated, as you can imagine, they had little idea that more heartbreak was on the way.
After Steven’s death, the Gunners activated an account at the Donor Sibling Registry, a non-profit that connects sperm and egg donors with donor-conceived individuals and families. Since Steven was conceived with donated sperm from Fairfax Cryobank’s donor 1558 and his mother’s egg, they hoped they might catch a glimpse of him in some of his donor siblings. They also felt an obligation to inform other parents and donor siblings that Steven, whom they loved with all their heart, had suffered from schizophrenia. As such, he was prone to addictions and erratic behavior and had been hospitalized on numerous occasions for mental health emergencies.
The biological father of Steven Gunner, who battled mental illness before a fatal overdose, had his own psychiatric problems
A Rochester doctor is accused of using his own sperm to inseminate a woman after telling the prospective parents that the sperm would come from a separate donor, a new lawsuit alleges.
"The lawsuit alleges medical malpractice against Wortman and several employees at the facility, claiming that Wortman knowingly implanted his own sperm in the plaintiff’s mother and later treated her [his biological daughter] from 2012 through 2021, including conducting numerous pelvic examinations, transvaginal ultrasounds, and IUC placements under sedation."
Both families are preparing to file 2 separate lawsuits against the University of Utah Center for Reproductive Medicine.
Families who claim disgraced Ottawa fertility doctor Norman Barwin used the wrong sperm — or even his own sperm — in the conception of at least 100 children could receive a portion of a multi-million-dollar payout after a judge certified the class-action lawsuit launched in 2016.
The Ontario Superior Court certified the class-action suit in the landmark case at a hearing on Wednesday, which includes a negotiated proposed settlement worth $13.375 million.
BRISTOL, CT — A Superior Court judge awarded a Bristol family $37.6 million in a medical malpractice lawsuit against the state in connection with an insemination procedure at the UConn Health Center that led to the death of one child and a lifetime of medical needs for her twin, according to the Hartford Courant.
The Courant reports Jean-Marie Monroe-Lynch and Aaron Lynch filed a lawsuit that claimed Monroe-Lynch was inseminated with sperm from a donor who carried CMV, a virus that can cause severe birth defects or fetal death if contracted during pregnancy.
One child died in utero in January 2015 and her twin brother sustained a brain injury that requires lifetime medical attention, according to the Courant.
June: Louisville Courier-Journal
Inseminated by doctor who used his sperm, woman says conduct 'unconscionable and depraved'.
LOUISVILLE, Ky. — Erin Crowder says her parents told her when she turned 18 her father had fertility problems and she was born after her mother was inseminated with sperm from an anonymous donor.
But Crowder, now 45, said it was only when she underwent a genetic test from Ancestry.com two years ago that she discovered the identity of her biological father.
Of all people, he was her mother’s Louisville fertility doctor, Dr. Marvin Yussman.
She learned as well she had at least seven half-siblings, all fathered by Yussman, who used his own sperm to inseminate patients.
Her mother, Susan Crowder, 73, formerly of Hopkinsville, said she was livid when she learned about she calls Yussman’s “unconscionable and depraved” conduct, which has come to be known as fertility fraud.
Bianca Voss, of Clifton, New Jersey, raised her daughter, Roberta, only knowing her father was an anonymous sperm donor that was allegedly selected by her fertility specialist, Dr. Martin Greenberg. “He did say, ‘Do you mind if the donor was Jewish?’ And I said no. I kind of thought it might be a medical student from the hospital, but that was it,” Voss said. Voss says at the time the doctor asked if she had a preference for ethnicity or religion. He charged a $100 fee to procure the sperm. But recently, daughter Roberta, now of Palisades, New York, learned from the DNA ancestry website 23AndMe that her father is Dr. Greenberg, himself. Back in 1983, Greenberg had a practice in an Upper East Side building. Today, he lives in Aventura, Florida, outside Miami.
“You’re playing Russian roulette with this,” says Cassandra Bach, a fertility coach and mother of a donor-conceived child. When she decided to get pregnant on her own via a sperm bank donor in 2010, she was told that sperm from the donor she chose wouldn’t be given to more than 40 families. But when her daughter was 2 years old, Bach joined the Donor Sibling Registry (DSR), a nonprofit that helps connect donor-conceived people with biological relatives (many parents join so there’s no chance of their child dating an unknown half-sibling). Over time, she discovered her daughter had 114 half-siblings spanning four countries on three continents. “The sperm bank was tracking siblings via self-reporting, and not everyone reports when they’ve had a child,” says Bach.
These are the reasons the industry needs massive reform, says Wendy Kramer, director and cofounder of DSR. Many parents of donor-conceived children are pushing for mandatory background checks on donors, as well as mandatory reporting about where else they’re donating and accurate tracking of the number of children created by donors. Clinics “need to enforce reporting from donors if their health history has changed,” says Kramer. “Many of the people who donate to these banks are 19-year-old kids, so you’re getting a snapshot of one day in the life of a healthy young person. His father could die of a heart attack the next year, or he could develop cancer later in life, and you wouldn’t know.” She’s been pushing for change for years now, but progress has been slow. “The thing is, it costs money to keep accurate records and to do proper vetting,” says Kramer.
One long-touted pro of using a sperm bank was the promise of donor anonymity. But the idea isn’t realistic anymore with at-home DNA testing kits, and, some experts argue, total anonymity is harmful for donor-conceived people anyway. In fact, 94 percent of donor-conceived people strongly support the option to access info about how many siblings they have and the identity of their donor, and 99 percent want details about the medical history of their donor, per a recent survey. “Kids will ideally find out they are donor-conceived early in life, or they will find out later through a DNA test or family member,” says Kramer. “Either way, they’ll have questions and may want to make contact, and all parties should understand and be open to that.”
The case against a retired Idaho gynecologist accused of using his sperm to inseminate a patient seeking fertility treatments has been dismissed.
Besides health concerns, there is another important reason for limiting donor’s fecundity. The children of sperm and egg donors, like those who are adopted, often want to trace their blood relations. But it is difficult to forge strong relationships when vast numbers of children are involved. Wendy Kramer of the Donor Sibling Registry, which helps connect members of donor families, says this is an example of how the contract between clinics and would-be parents has ignored the interests of the children it produces. She established the group in 2000 when her then ten-year-old son, conceived using donor sperm, became curious about his wider family. Last month he learned of the existence of two new half-siblings, bringing the tally to 22. Ms. Kramer had been told her sperm donor would father no more than ten children, a limit she considers sensible.
More than 100 women and men have begun a class action lawsuit against Monash IVF following claims the national fertility specialist may have inadvertently destroyed healthy embryos through a faulty genetic screening test.
Documents were filed in the Supreme Court of Victoria on Wednesday against Monash IVF and the Adelaide Fertility Centre on behalf of patients who attended the clinics between May last year and October this year.
"Dr. Philip Peven is credited with delivering around 9,000 babies during his 40-year career in Detroit, Michigan. Now a group of siblings who found they were genetically matched after doing an online DNA test have discovered they are related to Dr. Peven, who was their parents' doctor, and believe he is their father."
"Dr. Quincy Fortier ... over the course of decades impregnated an unknown number of female patients at his Women’s Hospital. [But] Fortier wasn’t simply a cretin who surreptitiously implanted innocents with his own sperm — he was a serial pedophile who preyed upon his own children, including one stepdaughter whom he personally impregnated."
"Traci wanted more medical history information, but quickly learned that in California, donor-conceived children have no rights to sue their biological parent."
NOTE from DSR: It's important for people to know that this phenomenon of doctors inseminating their own patients was a regular and common occurrence from (at least) the 1940s through the 1980s when all sperm was fresh, not frozen. Once cryopreservation and sperm banking came into place, this practice seems to have almost stopped (except for a couple of rogue doctors).
"A deceased gynecologist has fathered at least 17 children with women thinking they were receiving sperm from anonymous donors, a Dutch hospital said Tuesday, in the latest IVF scandal to hit the country."
"The Georgia Supreme Court on Monday decided claims that a sperm donor lied about his mental and criminal history led to damages in line with consumer fraud, but that 'life itself can never be an injury.'"
"He fathered at least 36 children, but the parents didn't know he had no education and a history of hereditary mental illness."
This story is an example of the UK not enforcing the "10 family limit." [Note: The DSR does NOT support this donor's main reason for suing, which was the fact that the clinic sold his sperm to gay couples and single mothers. We do, however, recognize that the clinic did not limit the number of children created from the donor's sperm.]
"A fertility doctor could have fathered hundreds of children, secretly using his own sperm instead of the donor sperm his patients thought they were receiving."
"A San Diego County woman filed a lawsuit Wednesday, alleging that a local physician she consulted for fertility issues decades ago used his own sperm to artificially inseminate her, only discovering the fact after her adult son took a DNA test."
"Multiple families victimized by 'fertility fraud' took action today by filing cases against doctors in California and Virginia/West Virginia. The doctors are alleged to have inserted their own sperm into their patients, contrary to the doctors’ representations and their patients’ wishes."
"On Wednesday, September 2, the NW Cyrobank in Spokane, which acquired donor vial inventory and records of Cryo LLC in 2016, sent an email to families and revealed that donor 518 and 901A were the same person. The mother we spoke to told us that the donor is believed to have fathered children across Washington, Idaho, and Montana and that some of the kids have health problems such as autism, ADHD, anxiety, and her son has hemophilia, a rare blood disease that can be passed on by a father."
"With little regulation in the sperm bank industry, stories of mistakes and sloppy record-keeping are growing. It’s blowing up the lives of donor-conceived children."
"A Georgia sperm bank claims no accountability for selling sperm of man with a criminal history and a diagnosis of bi-polar with schizo-affective disorder.
In the end, Nahmias summed up the debate asking, 'just to be clear, what you’re asserting, a sperm bank can completely misrepresent everything about the sperm itself and charge whatever amount of money based on those representations and completely lie to every customer it has - and nobody can do a thing about it?'
Xytex response was under the law, yes.
That’s why 38 law professors from across the United States filed their own legal analysis with the court asking justices to allow the Normans’ lawsuit, and others like it, to go to trial.
In the brief, written by Georgia State University College of Law Professor Timothy Lytton, they argue, '“exposing sperm banks to liability will give them a powerful incentive to exercise reasonable care in vetting donors and providing accurate information to their clients.'”
Legal Issues

Lawsuits
Family alleges artificial insemination mix-up after 30 years, sues hospital.
Former fertility patients of Dr. Nicholas J. Spirtos at Summa Health System filed a major new lawsuit after a shocking revelation was discovered by an Ancestry.com DNA test. The couple had undergone an insemination procedure intended to fertilize the wife’s egg with the husband’s sperm. But due to an apparent mix-up of sperm samples, their daughter is not biologically related to the man who raised her. Attorneys for the family at the law firm Peiffer Wolf Carr Kane Conway & Wise (Peiffer Wolf) said further testing indicated the sperm came from a different patient of Spirtos.
At least 100 people have been found to have been conceived using the wrong sperm while being treated by Dr. Barwin, at least 17 of whom were conceived through the use of his own sperm. Dr. Barwin's medical license was revoked in 2019, previously having been suspended in 2013 for the same conduct, and again in 2014.
In July 2021, an Ontario court certified a class action suit against Dr. Barwin for battery, breach of trust, breach of contract, negligence, and other torts. The class includes some 226 plaintiffs, including the aforementioned people conceived by Dr. Barwin using the wrong sperm, as well as patients who were wrongly inseminated with the sperm, and patients whose sperm was wrongly used to conceive someone else's child. The same month, the plaintiffs and Dr. Barwin came to a proposed settlement that has since been approved by the court. The settlement is considered to be the first of its kind, not only in Canada but internationally (although unfortunately, Dr. Barwin's actions in using the wrong sperm are not unique).
The reason that the proposed Barwin settlement is so significant is twofold: It is a large class action settlement (more than 13 million Canadian dollars), and some of the settlement funds would be directed to create a private DNA database.
Dr. Morris Wortman is being sued by his alleged biological daughter, Morgan Hellquest.The lawsuit says in 1983, the plaintiff's mother Mrs. Levey, became a patient of Dr. Morris Wortman at his OB/GYN medical practice in Rochester. The practice offered fertility medicine to patients trying to conceive a child.
In late 1983 through January 1985, the lawsuit says Wortman inseminated Mrs. Levey two to three times monthly in either his office or at Highland Hospital in exchange for Levey's payment of $50.00 in cash or check for each insemination. The suit claims Levey was under the impression she was receiving an anonymous medical student’s live sperm. In January 1985, Wortman inseminated Levey with a live sperm she became pregnant.
Hellquest says through DNA testing, she later discovered that she shared DNA with Wortman’s daughters and had six half-siblings with the same father.
The suit also claims Wortman has a family history of mental illness, despite telling Hellquest's mother and step-father that the true father would have a clean health history.
BRISTOL, CT — A Superior Court judge awarded a Bristol family $37.6 million in a medical malpractice lawsuit against the state in connection with an insemination procedure at the UConn Health Center that led to the death of one child and a lifetime of medical needs for her twin, according to the Hartford Courant.
The Courant reports Jean-Marie Monroe-Lynch and Aaron Lynch filed a lawsuit that claimed Monroe-Lynch was inseminated with sperm from a donor who carried CMV, a virus that can cause severe birth defects or fetal death if contracted during pregnancy.
One child died in utero in January 2015 and her twin brother sustained a brain injury that requires lifetime medical attention, according to the Courant.
IVF patients across Australia fearing they've lost their only chance at having biological children are suing a major provider over the destruction of embryos that may have been viable. Monash IVF is at the centre of a lawsuit filed in Victoria's Supreme Court on Wednesday, over a world-first non-invasive genetic testing program that has now been suspended. More than 100 people have already expressed interest in the action but upward of 1000 could be affected, lead lawyer Michel Margalit told AAP.
From Molly McCafferty, lawyer and DSR Board member: "The court denied California Cryobank’s motion to dismiss, allowing Branzell to proceed with his case. California Cryobank was trying to get the case dismissed from federal court on a technical basis — arguing that all parties were from the same state and so the federal court did not have jurisdiction to hear the case. The court has ruled that the parties can engage in discovery to resolve this issue. After discovery is conducted, California Cryobank could attempt again to dismiss the case on these grounds." January 2021: Bryce Branzell settled with California Cryobank with prejudice, which means Branzell cannot attempt to bring claims against California Cryobank in the future. The settlement amount remains undisclosed for now.
"In 1988, Plaintiff and her husband turned to Defendant for fertility treatment. Without Plaintiff’s knowledge or consent, Defendant used his own sperm to impregnate her, rather than, as promised, sperm from an anonymous donor."
"In 1978, Plaintiff and her husband turned to Defendant for fertility treatment. Without Plaintiff’s knowledge or consent, Defendant used his own sperm to impregnate her, rather than, as promised, sperm from an anonymous donor."
Wendy Norman et al. v. Xytex Sperm Bank. Supreme Court of Georgia BRIEF OF AMICI CURIAE LAW PROFESSORS OF TORT LAW, FAMILY LAW, AND HEALTH LAW IN SUPPORT OF PLAINTIFF-APPELLANTS.
September 2020 Update: Georgia Supreme Court Rules Sperm Donor Case Falls In Line With 'Consumer Fraud'
"The Georgia Supreme Court on Monday decided claims that a sperm donor lied about his mental and criminal history led to damages in line with consumer fraud, but that 'life itself can never be an injury.'"
Branzell v. California Cryobank/NW Cryobank. "The acts of NW Cryobank have resulted in one individual who did not want to be a sperm donor having at least one child conceived by artificial insemination. Worse still, NW Cryobank cannot determine how many others were provided sperm from Branzell, or continue to be provided his sperm. Branzell chose not to become a sperm donor because he did not want children he had no relationship with. It has now been confirmed that Branzell is the genetic father of a child he has never met. Branzell is now haunted by what he does not know, specifically how many other mistakes NW Cryobank made, and how many other children were or will be conceived with his sperm." January 2021: Bryce Branzell settled with California Cryobank with prejudice, which means Branzell cannot attempt to bring claims against California Cryobank in the future. The settlement amount remains undisclosed for now.
Teuscher v. CCB-NWB: Lawsuit against California Cryobank/NW Cryobank. "NW Cryo has engaged in a series of deceptive business practices...." Amended Complaint, filed August 12, 2019. Exhibit A; Exhibit B; Exhibit C. Second Amended Complaint, filed November 12, 2019. October 2019 Above the Law article: Beware Of The Home DNA Test! Mom Strikes Back Against Sperm Bank. December 2019: Plaintiff's Opposition to Defendant's Partial Motion to Dismiss: NW Cryo has brought a second motion to dismiss some claims in the lawsuit: the consumer protection claim, deceptive and unfair practices including confiscating Danielle Teuscher's gametes — the gametes that would help to make a genetic sibling for her daughter, re-classifying donor from Open ID to anonymous, taking down the sibling registry it maintains, and withholding access to medical information of donor, the declaratory judgment claim (contracting with customers against the public policy of the state of Washington) and Ms. Teuscher's intentional emotional distress claim for NW Cryo's retaliatory and intentional actions of sending a Cease and Desist letter and threatening to file a lawsuit against her and illegally taking away her gametes. Ms. Teuscher and her daughter's team of lawyers, led by Law Offices of Jill H. Teitel, PLLC are working to fight the baseless motion. February 2020 Above the Law article: Sperm Bank Case Has Serious Implications For Information and Property Rights. November 2020 Update: This lawsuit was settled out of court for $75,000.December 2020: read the case summary with Q & As by Jill H. Teitel, Esq. attorney for Danielle Teuscher and Z.F.
OSHU Lawsuit: Dr. B.C. aka Dr. John Doe v. Oregon Health & Science University. An Oregon doctor who says he discovered that his donated sperm was used to father at least 17 children in violation of an agreement that allowed for the creation of no more than five children filed a $5.25 million lawsuit against Oregon Health & Science University. Oregon doctor says his donated sperm was used to father at least 17 children, sues OHSU for $5.25 million
Vidiksis v. California Cryobank: Class Action Complaint filed against California Cryobank for conversion of and tortious interference with donor sperm from Manhattan Cryobank. From the attorney: "Hi Wendy. I hope you are doing well. Would you mind letting your followers know about the new class action we filed last Friday against California Cryobank? Basically, California Cryobank purchased some assets of Manhattan Cryobank, including all donor sperm. Many people have purchased donor sperm from MCB and were storing it with MCB in hopes of conceiving a second child with the same donor sperm. Now, CCB will not give them access to the donor sperm, claiming that the FDA will not let them release it for some unknown reason."
Frankiewicz and Perez v. Manhattan Cryobank: Lawsuit against Manhattan Cryobank/California Cryobank. From the attorney: "For years, Defendant MCB sold sperm to the public which it knew could contain genetic diseases. Worse, MCB’s own chairman of the board recognized the inadequacies of MCB’s genetic testing regimen and touted the benefits of a more robust screening technology. Nonetheless, MCB, despite having access to such technology, chose not to use it on sperm donated prior to November 1, 2014, despite agreeing and warranting to its customers that it performed a 'complete and thorough screening' for genetic diseases. Read the NY Post article: Manhattan sperm bank didn’t properly screen for genetic diseases: suit
Jane Doe 1 and 2 v. Xytex: Wrongful birth lawsuit against Xytex. Xytex sperm donor #9623 was a mentally ill schizophrenic felon. In April 2016, other parents who had used Xytex donor #9623 filed a complaint in San Francisco County Superior Court, alleging "intentional misrepresentation, negligent misrepresentation, strict products liability, products liability based on negligence, breach of express warranty, breach of implied warranty of merchantability, battery, negligence, false advertising, wrongful birth, specific performance, punitive damages, and violations of the California Unfair Competition law."
Jacoba Ballard and Deborah Pierce v. Anonymous Health Care Provider d/b/a Anonymous and John Doe, M.D. Dr Donald Cline of Indianapolis used his own sperm to inseminate patients and father more than 50 children. "Specimens from a single donor were to be used in no more than three successful artificial insemination procedures in a well—defined geographic area. Therefore, specimens from a single donor were not to be used in more than three successful artificial insemination procedures at Anonymous. 34. The policy described in paragraph 33 was important to limit the risk of accidental incest resulting from many closely biologically related individuals living near each other and unaware of their biological relationships. 35. At no time did John Doe, M.D. ever disclose to any patient that he would use his own sperm to inseminate them.
Jane Doe v. Idant Laboratories. "The boys are part of an autism cluster involving at least a dozen children scattered across the United States, Canada, and Europe, all conceived with sperm from the same donor. Many of the children have secondary diagnoses of ADHD, dyslexia, mood disorders, epilepsy and other developmental and learning disabilities."
Jane Doe v. NECC. "Jane Doe, the plaintiff, brought this equity action against the defendants New England Cryogenic Center, Inc. (NECC), and its director, John Rizza (Rizza), seeking an order requiring them to disclose the name of a sperm donor reflected on NECC's records as donor number D237 and whom we shall call D237. Doe claims that she was artificially inseminated in London, England, with D237's sperm and consequently bore twin daughters."
Donna Donovan v. Idant Laboratories. In 1995, Donna Donovan was artificially inseminated with sperm provided by Idant Laboratories. Ms. Donovan signed a consent form in which Idant represented that “(1) semen stored at Idant is exceptionally safe; (2) Idant has a screening program that far exceeds mandated standards and (3) Idant’s donors go through a rigorous screening process to ensure that they have a good genetic background and history.” Donovan gave birth to a daughter, Brittany, in January 1996 using sperm from Idant Donor G738. Brittany was diagnosed with developmental difficulties related to her status as a carrier of the Fragile X gene (FMR1). Testing revealed that Donor G378, not Donna Donovan, carried the Fragile X gene.
Johnson v. California Cryobank: Lawsuit against California Cryobank. "[CCB] failed to disclose that the sperm they sold to the Johnsons came from a donor with a history of kidney disease called Autosomal Dominant Polycystic Kidney Disease (ADPKD). That sperm was used to conceive [their daughter] Brittany who has been diagnosed to have this serious kidney disease."
United States of America v. Cecil B. Jacobson, Jr., M.D., Case No. 92-5406 and Cr. No. 91-00474-A, 785 F. Supp. 563. "Dr. Cecil B. Jacobson was indicted on fifty-three counts of mail fraud, wire fraud, travel fraud and perjury. In the indictment, the Government alleges that Dr. Jacobson defrauded certain women and their husbands by representing that the women would be inseminated with sperm from an anonymous donor participating in a donor insemination program. The Government further alleges that, contrary to these representations, Dr. Jacobson inseminated these women with his own sperm, thereby becoming the biological father of the children born to certain of his patients."
2017 FDA Citizen's Petition
July 2017 FDA Citizen's Petition: "Request that the FDA look into the state of affairs surrounding the sperm donation industry, and then develop the appropriate and much-needed regulation/oversight." Read the 173 associated comments, stories, and powerful testimonials. Here is a PDF of the August 9, 2018, Letter of Denial. (The FDA will only deal with "communicable disease" such as STDs in gamete donation.)
ASRM "LIMITS"
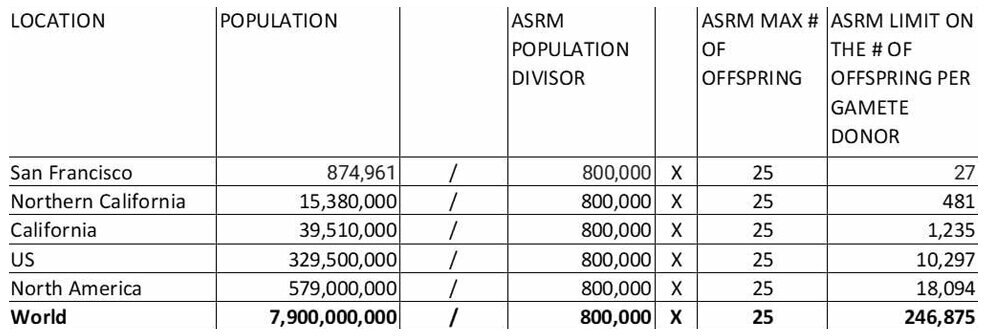
From Marilyn Huff: ASRM trick formula for unlimited Profits selling unlimited offspring per donor to maximize the profit of physicians and cryobanks
The American Society of Reproductive Medicine came up with guidelines that sound like a limit without actually being a limit; it's a formula that limits donor use to no more than 25 pregnancies per donor in a population of 800,000. There is no limit, it's a trick: Divide the population of any city, state, country by 800,000 then multiply by 25 to get the number of offspring the donor is allowed to have in that city, that state, that country, etc.
Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)
Wendy received this email from a DSR member, who used The Sperm Bank of California (TSBC): "The sperm that we purchased years ago to continue growing our family has been labeled 'not usable' by the FDA because our donor had had sex with men."
The DSR is aware of plenty of gay donors, so we wondered why TSBC would deem $10,000 worth of sperm unusable when they knew the donor was gay when he donated? This 10/19 "Warning Letter" from the FDA to TSBC gives some insight.
Upon investigation, we found the (2007) FDA's Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps), which states: "In accordance with § 1271.75(d), you should determine to be ineligible any potential donor[s] who ... have had sex with another man in the preceding 5 years."
Molly McCafferty, a lawyer and DSR Board member, provided this clarification:
"I am not aware of any laws or binding federal regulations banning gay donors.
In 1983, the FDA issued a ban on sperm donation from men who had had sex with men in the preceding five years. In 2005, the FDA updated its guidance on this issue, which many view as encouraging sperm banks to avoid donations from men who have had sex with men.
Under non-binding recommendations issued by the FDA in 2007, men who have had sex with men in the preceding five years is included in a 'list of conditions and behaviors that increase the donor’s relevant communicable disease risk ... you should determine to be ineligible any potential donor who exhibits one or more' of these 'conditions or behaviors.' (See p. 14.) Again, these are non-binding and are recommendations only.
As you know, under current FDA regulations, all sperm must be tested for HIV (among other communicable disease, both sexually and non-sexually transmitted (e.g., Hepatitis B and C). As you also know, American sperm banks typically freeze donations for a minimum of six months before insemination, during which the semen is tested. Obviously if semen tested HIV+ it would be ineligible whether the donor had had sex with men or not. The FDA seems to have some old 'recommendations' implying that men who have sex with men are at higher risk for transmitting communicable diseases (as with blood donation), which may encourage sperm banks to screen out men who have had sex with men in the preceding five years out of some idea of an abundance of caution before getting to the testing for communicable diseases stage. As all sperm must be tested and quarantined regardless of sexual history, this obviously makes no sense. But of course private sperm banks are unfortunately free to admit and deny anyone they like as sperm or egg donors (e.g., setting age limits, educational criteria, height requirements, etc.).
21 CFR §1271 Subpart C contains Federal Regulations regarding donor eligibility. Federal regulations do carry the same authority as federal law. Per these regulations, a donor is eligible if donor screening done in accordance with FDA regulations indicates that the donor is free from risk factors for, and clinical evidence of, infection due to relevant communicable disease agents and diseases and the results of donor testing for relevant communicable disease agents are negative or nonreactive. As I read these, as long as semen tests negative as to the communicable diseases listed in the FDA guidelines, it is eligible sperm — no matter from whence it came. If the sperm tests positive for communicable diseases listed in the FDA guidelines, it is ineligible sperm — no matter from whence it came. There is a difference between screening potential sperm and testing potential sperm, but [this] case is especially strange as the sperm had already been sold."
NY State Law
December 2021: Senate Bill S76023 introduced to the NY State Senate: The donor-conceived person protection act.
Sperm banks/clinics would be legally required to collect medical, educational, and criminal felony conviction records from donors, use them to verify some of the medical, educational, and criminal felony information self-reported by donors, and disclose what information is verified or not verified to recipient parents. The bill would be a small step toward greater regulation/accountability.
NYCRR Title 10 PART 52-8.9 Required records.
(a) Reproductive tissue bank records shall be open to inspection by the department and shall be kept for at least seven years after release of reproductive tissue for artificial inseminations or assisted reproductive procedures not resulting in a live birth, and 25 years for inseminations or assisted reproductive procedures known to have resulted in a live birth. For all donated reproductive tissue, the donor's name, address, and any other information which would directly or indirectly identify the donor shall not be disclosed or released by the reproductive tissue bank to any person or entity, except upon the written informed consent of the donor, or except to authorized employees of the department or as permitted by law. The recipient's name, address, and any other information which would directly or indirectly identify the recipient shall not be disclosed or released by the insemination/implantation site to any person or entity, except upon the written informed consent of the recipient, or except to authorized employees of the department, or as permitted by law.
(b) In addition to the recordkeeping requirements of section 52-2.9(c) of this Part, each reproductive tissue bank shall maintain applicable donor/client-depositor records which include:
(1) for donors, pertinent family history of any genetic disorders;
(2) documentation of donor and client-depositor written informed consent;
(3) for semen donors, outcome of any prior artificial insemination or other assisted reproductive procedures, if known, including number of successful pregnancies, if any, and any reports from insemination/implantation sites which would affect the donor's acceptability; and
(4) documented approval of the reproductive tissue bank director, or his/her designee, of the acceptability of the donor.
(c) In addition to the recordkeeping requirements of section 52-2.9(e) and (f) of this Part, each reproductive tissue bank shall maintain applicable records which include:
(1) donor's identification code or client-depositor's name;
(2) for semen donations, documentation of laboratory cryosensitivity testing, and, if performed, results of viability checks after thawing and during storage, if any;
(3) the name of the insemination/implantation site, the physician or other person authorized by law to perform artificial insemination or assisted reproductive procedures, and/or receive reproductive tissue, and the name of the person communicating the order for distribution of the tissue;
(4) the recipient's name, if the name has been provided to the reproductive tissue bank with her informed consent, or the recipient's identification code, if used;
(5) documentation of training, certification, licensure, if required by law, and continuing education for each staff member; and
(6) any adverse outcomes, including infectious diseases in recipients or their offspring and genetic defects in offspring, which shall be reported to the donors if there is any possibility that the donor's reproductive tissue contributed to the adverse outcome.
(d) The following records shall be kept, separate from the recipient's records, by an insemination/implantation site for each insemination or assisted reproductive procedure performed:
(1) donor's identification code or name, if the reproductive tissue originates from a client-depositor;
(2) evidence that reproductive tissue from donors and/or client-depositors has been obtained from a reproductive tissue bank licensed pursuant to Subpart 52-2 of this Part;
(3) disposition of the reproductive tissue, including, but not limited to, the name or identification code of the recipient, destruction logs, and autoclaving or incineration records;
(4) the name and signature of the ordering physician or other person authorized by law to order issuance of the reproductive tissue;
(5) results of sperm viability checks, if performed; and
(6) signature of the person receiving the sample and condition of the sample upon receipt.
(e) The insemination/implantation site shall document the outcome of the artificial insemination or assisted reproductive procedure, including, but not limited to, any known adverse outcome in the infant or infectious disease in the recipient, as well as any known successful pregnancies. This information shall also be reported to the reproductive tissue bank releasing the tissue, even if the reproductive tissue bank is the same entity as the insemination/implantation site.
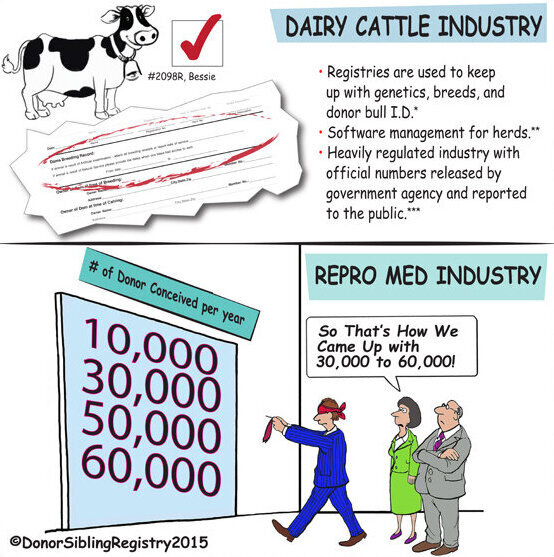


Sperm Bank Policies: What are they thinking?

General Policies
2025: Xytex, From a DSR member:
"Hi Wendy - I wanted to pass along a message for the whole community about something that Xytex and I guess other sperm banks are doing that people shouldn't accept.... I recently tried to ship sperm from Xytex that I've stored for over 10 years, and usually, when I ship, the only document I needed to sign was a shipping agreement covering the shipping charges and details etc. This time, however, they sent me a docusign that had loaded into it various other agreements like a Services agreement and others that appeared to be renegotiating the terms under which I hold the sperm at Xytex - these terms I agreed to 10 years ago when I initially bought the sperm and paid each year to store it. Those sperm belong to me, and I have paid Xytex for storage; they should not be holding it hostage unless I sign additional legal agreements with them. I wanted to give a heads-up to the rest of the donor sample purchasing community that this is an outrageous attempt by Xytex to try to CYA and change their terms and conditions when those were already agreed to when I initially purchased the sperm. If they want to change T&C, the new ones can apply to new customers but shouldn't be imposed on the old ones. For them to "slip" them into the docusign that customers think they are signing for shipping alone is outrageous and dishonest. Very upset over this."
2024: HFEA/London Sperm Bank
When posting on the Donor Sibling Registry to connect with your own or your child's genetic relatives, it is important to post under the originating sperm bank/facility (where the donation occurred), as the large US (and Danish) sperm banks ship to hundreds of small clinics worldwide.
Recent reports have shown that some local facilities, including the London Sperm Bank in the UK and City Fertility Center in Australia, are reselling sperm from larger sperm banks (e.g., Seattle Sperm Bank and Fairfax), assigning completely different donor numbers in an attempt to obfuscate donor origins.
If you used a local clinic, please make sure that they did not buy the sperm from a large US (or Danish) sperm bank and then change the donor ID/number. Facilities that buy sperm from large sperm banks should share all original sperm banks and donor numbers. Covering up the origin of the sperm and the unidentifiable donor ID keeps people from connecting with half-siblings and donors and, therefore, also prohibits the sharing of important medical information with each other. Since the sperm banks have inaccurate records regarding the children born from any one donor, in most cases, the best way to connect, update, and share information directly with the families is through the Donor Sibling Registry.
Representatives from the London Sperm Bank pointed me to HFEA 2010 guidelines as to why they have deliberately hidden the non-identifying origins of their imported sperm and why they won't contact those families (who have no idea that their donors were not from the London Sperm Bank) to notify them of the correct sperm bank/donor ID. They put the onus on the families to ask, but you can't ask about what you don't know to ask about. It's simply lying by omission.
One argument that the London Sperm Bank folks made in defense of this practice was that "a study" showed that donor-conceived people do not wish to know their close genetic relatives. That's just not an accurate or logical argument. They also said they won't educate their parents and donors about the Donor Sibling Registry, again citing HFEA rules. I found nothing in the HFEA guidelines that backs up that claim.
The HFEA argues that donor numbers are identifiable information. "...donor codes have the potential to identify individual donors, and the sole purpose of disclosing donor codes to parents of donor conceived children would be to identify donor conceived siblings. As such, the release of donor codes would not fall within any of the relevant exceptions which permit disclosure of register information. The Authority has therefore determined that it will stop the disclosure of donor codes on a permanent basis, and strongly advises all centres licensed by it to take immediate steps to ensure that they have systems in place to prevent the unlawful disclosure of donor codes either directly on request, or indirectly. This would include measures such as the redacting of patient records where required, and making any necessary modifications to witnessing arrangements." That's a lot of effort to try and keep donors, donor-conceived people, and their families from making their own mutual consent contact at any time on the DSR.
Two weeks ago, I wrote to the HFEA's Chief Executive, the Director of Compliance and Information, and another contact who is an HFEA Authority member to ask them about the 14-year-old guidelines. No one replied. The UK, which some consider progressive in how it deals with donor conception, seems unwilling to adequately counsel and educate parents and donors on the importance of a child connecting early in life (long before 16 or 18) with their siblings and, yes, even with their biological parents. There are many published research studies and 24 years of DSR stats, stories, and anecdotal information from almost 100,000 people that provide this evidence.
The HFEA is, in effect, enforcing out-of-date guidelines that try to keep donor-conceived people from their close genetic relatives, by mandating the deliberate changing of donor numbers and by not allowing sperm banks to contact all affected families to notify them of the correct originating facility and donor number. Since 2005, donor anonymity has been an illusion anyway. They need to start riding the horse in the direction it's going.
2021 Fairfax Cryobank unenforceable and threatening contract verbiage:
"What Client Agrees To Do: 3.1. Donor Anonymity. Client agrees to use the information Cryobank provides about donors, including photographs, exclusively for the purpose of selecting a donor, and not to share, distribute or otherwise make any such information or images available in any manner, or through any medium (including without limitation email, social media or internet feeds), to any third party. Client will not seek a donor’s identity. If Cryobank discovers that Client has made attempts to discover a donor’s identity, Cryobank will pursue any and all appropriate action to protect itself, its donors, and their other offspring."
How much do sperm banks make? Here's an interesting 2017 article from a marketing company that ran a marketing program for a New York sperm bank. "The cryobank gathered an additional 1,000 local leads during their seven-month trial. This resulted in an average lead growth of 166 percent month-over-month. After accounting for the number of leads that passed the sperm bank’s genetic testing, the resulting conversion rate was about 3 percent. In total, we earned the client an estimated $3,000,000 in additional revenue." They spent $21,000 on the ad campaign. So that means each approved donor will earn the sperm bank almost $100,000.
2017 book about the sperm banking industry: Scattered Seeds: In Search of Family and Identity in the Sperm Donor Generation. Journalist and writer Jacqueline Mroz looks at the growth of sperm donation and assisted reproduction and how it affects the children who are born, the women who buy and use the sperm to have kids, and the sperm donors who donate their genetic material to help others procreate. "This is the dawn of a challenging moment in time for assisted reproduction,” Mroz concludes. “With technology evolving rapidly, it’s time for legislators and policymakers to start acknowledging these changes and examine the consequences of an unregulated industry."
2017 quote from a donor-conceived person: "I don't think donor anonymity is an issue that is front of mind for the general public. It's individual fertility specialists and clinics seeking to retain control and living out their antediluvian paternalistic attitudes in practice that are the reasons behind the persistence of donor anonymity. They don't trust the lived experience of donors, donor-conceived people, or the parents of donor-conceived people and believe that in all cases "doctor knows best." When you have a combination of capitalism and patriarchy you're going to have problems with getting progressive values into place."
2016 interview with medical ethicist Art Caplan, Professor of Bioethics at NYU, Langone Medical Center: "Sperm Banks Are Run Like Grocery Stores From The 19th Century," Says Art Caplan
Cryogenic Laboratories Inc. implies that donor anonymity is required by law. A former sperm donor shared this photo of his CLI agreement. We call B.S. on CLI's terminology. There are no "legal statutes and standards" pertaining to donor anonymity. Also notice the typo ("statues") — who writes these illiterate, dishonest, misleading, and unenforceable sperm bank agreements?

Donor Anonymity

ALL donors in the US and Denmark are still sold as "anonymous" (be it for 18 years or forever), despite the fact that all donors can be found at any time via DNA testing and/or an Internet/social media/public records search.
The myth of donor anonymity is perpetuated by researchers. An article in the December 2016 issue of the Journal of Law and the Biosciences, Sperm donor anonymity and compensation: an experiment with American sperm donors, illustrates the fact that the US sperm banking industry is still not properly educating prospective donors and parents about the myth of anonymity. With DNA testing, Google, and social media, anonymity is a thing of the past. Sperm banks (and egg clinics) need to stop the fallacy of selling "anonymous" donors, and the "experts" need to stop perpetuating this idea. Donor-conceived people have been locating their donors via DNA testing since 2005 (see November 2005 New Scientist Magazine) — so this is not new. This published "study" was conducted in partnership with a sperm bank that profits more from offering anonymous donors. Communication with their donors about anonymity had already taken place. How was this major conflict of interest acceptable to the Journal of Law and Biosciences? In response to the article, Harvard Law published Wendy's blog contribution, DNA: Donors Not Anonymous, and the link to her HuffPo response blog, Sperm And Egg Donation: 10 Things Your Doctor, Clinic Or Sperm Bank Won’t Tell You.
The myth of donor anonymity is also perpetuated by infertility organizations, sperm banks, egg clinics, government organizations, etc. Why don't organizations where people go for support and guidance in the US, Canada, Australia, the UK, Europe, and other countries (e.g., the ASRM, HFEA, Path2Parenthood, Fertility Network, Donor Conception Network, BFS, etc.), along with all sperm banks and egg clinics, clearly educate on their websites about the fact that anonymity can no longer be guaranteed/enforced, not even if only for 18 years. They've all had since 2005 to disseminate this information, so it isn't something that they're just hearing about.
A letter from the Editor-in-Chief of Human Reproduction: "Due to genetic testing donor anonymity does no longer exist. Many thousands of people worldwide have been conceived with donor gametes but not all parents tell their children of their origin. Genetic testing will make this impossible. Over three million people have already used direct-to-consumer genetic testing. The rapidly increasing availability of cheaper and more detailed tests poses numerous challenges to the current practice of sperm and egg donation: 1. Whether they are donating in a country that practices anonymous donation or not, donors should be informed that their anonymity is no longer guaranteed, as they may be traced if their DNA, or that of a relative, is added to a database. 2. Donor-conceived adults who have not been informed of their status may find out that they are donor-conceived. 3. Parents using donor conception need to be fully informed that their children’s DNA will identify that they are not the biological parents and they should be encouraged to disclose the use of donor gametes to their children. All parties concerned must be aware that, in 2016, donor anonymity has ceased to exist." —JLH (Hans) Evers, Editor-in-Chief, Human Reproduction
Where around the world is donor anonymity banned? Austria, Finland, Germany, The Netherlands, New South Wales, New Zealand, Norway, Sweden, Switzerland, UK, and Australia. However, much of the sperm in these countries is imported from the US, which is still sold as "anonymous."


 Browse by Clinic
Browse by Clinic
 Become a Member
Become a Member
